
Innovative Strategies in the Prevention and Treatment of Peri-implantitis
Dentistry收到 24 Mar 2025 接受 08 Apr 2025 在线发布 09 Mar 2025
Focusing on Biology, Medicine and Engineering ISSN: 2995-8067 | Quick Google Scholar
END
Submit Article


收到 24 Mar 2025 接受 08 Apr 2025 在线发布 09 Mar 2025
Background: The field of dental implantology has recently experienced significant progress, transforming the approach to replacing lost teeth. Dental implants provide a reliable and long-lasting option for individuals seeking to regain their oral functionality and enhance their visual appeal. Nevertheless, obtaining successful results in dental implant procedures necessitates a thorough comprehension of the different factors that affect the success of the implants.
Objective: This manuscript aims to summarize current clinical insights and technological advancements in preventing and managing peri-implantitis. The paper highlights the importance of minimally invasive techniques, combined with augmentation procedures, in the gap between the implant and the alveolar walls, as this can lead to osseointegration and long-term stability of peri-implant hard and soft tissues. Additionally, it highlights the integration of digital tools such as 3D navigation and AI-assisted planning for accurate implant positioning and prosthetic design. This includes employing advanced materials, such as nano-coated implants and nanoparticles incorporated into artificial bone, as it boosts osseointegration and minimizes the likelihood of infection and inflammatory reactions.
Conclusion: Achieving a holistic understanding of thorough diagnostic assessments, modern surgical techniques, and advanced digital workflows is essential for successful implant therapy and the prevention of peri-implantitis. Further research and clinical application of nanotechnology promise to improve implant stability and longevity. Such an approach will enhance patient outcomes and quality of life.
It is estimated that around 12-18 million implants are sold annually globally. There is a high likelihood that a patient visiting a dental practice will have at least one dental implant in situ is high []. This reinforces the need for general dentists to acquire the knowledge and skills to diagnose and appropriately manage disease around implants.
Peri-implantitis is characterized by a destructive inflammatory lesion of polymicrobial etiology that affects both soft and hard tissues, leading to progressive peri-implant bone loss and the formation of a pocket and inflammation in peri-implant tissues. Modern dentistry is undergoing rapid changes due to the introduction of innovative technologies, particularly in implantology. This has underscored the need for a comprehensive approach to preventing and treating periodontal diseases, particularly with the use of new materials that promote implant osseointegration [,].
Peri-implantitis is an inflammatory disease that develops due to infection of the tissues around the implant. The main factors (Figure 1) contributing to its occurrence are:
Clinical manifestations of peri-implantitis include swelling, redness, bleeding gums, pain when touched and during chewing, and gradual bone loss. These symptoms can range from mild, indicating an early stage of the disease, to severe, where bone loss becomes critical to implant stability [].
Classification of periodontal and peri-implant diseases
For a comprehensive approach to diagnosing and treating peri-implant diseases and conditions, it is essential to have a clear understanding of their classification []. According to the American Dental Association (ADA) and the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), peri-implant diseases are categorized under diagnostic codes K08.81 (Peri-implantitis) and K08.89 (Other specified disorders of teeth and supporting structures), supporting accurate clinical documentation and classification [,].
According to ‘The 2017 World Workshop’, peri-implant diseases, in general, can be divided into four major categories (Figure 2) [].
Peri-implant mucositis is characterized by clinical signs of inflammation around the soft tissues surrounding a dental implant without signs of progressive marginal bone loss of more than 2 mm after supra-structure installation [].
Peri-implantitis is characterized by inflammation around the peri-implant tissues, typically with deepening of probing pocket depths concomitantly with progressive supporting bone loss (more than 2 mm) beyond the initial bone remodeling phase after prosthesis installation. Without previous clinical and/or radiographic records, peri-implantitis can be defined as the combination of ≥ 3 mm bone loss from the crestal bone level and ≥6 mm probing depths with bleeding or suppuration [].
As mentioned above, peri-implantitis is a considerable biological complication with unpredictable progression rates. Therefore, prevention is key to reducing the risk of peri-implant diseases. Recently, an Implant Disease Risk Assessment (IDRA) tool [] was developed by Heitz-Mayfield and co-workers to determine a patient’s risk profile in developing peri-implant diseases []. Eight parameters are proposed, each with criteria categorizing low, medium, or high risk. As a result, the tool determines the patient’s overall risk profile. Generally, a high-risk patient is categorized as having at least two parameters in the high-risk category. A low-risk patient has all parameters in the low-risk category, or at most, one parameter is categorized as moderate risk []. The tool helps guide clinicians on the need to modify higher-risk parameters. It is important to note that one non-modifiable parameter is a history of periodontitis. Therefore, identifying this cohort of patients with implants is crucial, as the criteria to be labeled low-risk are more stringent.
With the ever-increasing demand for implant placement to restore edentulous sites, the prevalence of peri-implant diseases is expected to increase. The first step in appropriately managing such diseases is correctly diagnosing peri-implant health, peri-implant mucositis, or peri-implantitis []. This is particularly important for patients with a history of periodontitis with a higher risk of peri-implant disease [,]. As a result, prevention of peri-implant diseases is imperative, particularly for high-risk patients.
Diagnosis is attained through probing, appreciation of clinical signs such as bleeding and/or suppuration, and radiographic assessment. Additionally, using the IDRA tool will guide clinicians in highlighting higher-risk patients requiring more stringent and closer follow-up.
A clinician’s expertise is pivotal for the success of dental implants and the prevention of peri-implantitis. The success of implant therapy goes further than just choosing high-quality materials; it requires meticulous planning, precise surgical techniques, and navigating complex anatomical structures using advanced tools such as 3D imaging and computer-assisted navigation [].
Clinicians play a crucial role in ensuring implant success and preventing peri-implantitis by integrating several key aspects into their practice. Comprehensive treatment planning is essential, beginning with a thorough assessment of the patient’s medical history and risk factors. Advanced imaging techniques, such as CBCT scans [], allow for precise evaluation of bone quality and quantity [,]. Adopting a prosthetically driven approach [], clinicians can optimize both function and esthetics when determining implant positioning [].
Surgical expertise is equally important in achieving long-term success. Atraumatic extraction techniques and ridge preservation help maintain bone volume, while careful flap design and suturing promote optimal soft tissue healing []. Minimally invasive techniques reduce postoperative complications and enhance recovery and overall outcomes [].
Integrating 3D navigation [] and digital workflows has revolutionized implantology []. Computer-guided surgery allows for more precise implant placement and reduces surgical risks [], while digital impressions ensure accurate prosthetic design []. AI-assisted planning [,] adds another layer of predictability and safety, improving overall treatment success [,].
Managing peri-implant soft tissues and occlusion is critical to reducing the risk of complications. Proper contouring of the surrounding tissues helps minimize plaque accumulation, while careful occlusal adjustments prevent excessive forces on the implant. Selecting biocompatible materials further promotes tissue health [] by minimizing inflammatory responses []. Precise surgical placement of dental implants is vital for osseointegration, the process through which the implant fuses with the surrounding bone.
Long-term maintenance and patient education are fundamental to implant longevity. Establishing personalized hygiene protocols ensures patients can effectively care for their implants []. Regular follow-ups, including radiographic and clinical assessments, help detect potential issues early. Additionally, timely intervention in cases of peri-implant mucositis can prevent progression to more severe complications [] (Figure 3).
By combining these principles, clinicians can significantly enhance the success of dental implants while reducing the risk of peri-implantitis, ultimately improving patient outcomes and quality of life [].
The modern development of nanotechnology allows the creation of materials that significantly increase the effectiveness of implantation and reduce the risk of peri-implantitis. The following innovative approaches are particularly noteworthy:
The use of titanium implants with hydroxyapatite nanoparticles promotes better osseointegration. Nanocoating creates an optimal surface for bone cell adhesion, which provides a stronger connection between the implant and the bone. In addition, some nanocoatings have antimicrobial properties, which reduce the risk of bacterial contamination.
Nanoparticle coatings and nano-structuring advancements enhance implant osteointegration and functionality []. These nanotech applications can create organized (isotropic) or disorganized (anisotropic) surface patterns, with dental implants often using anisotropic templates because of structural complexity. Titanium dioxide nanoparticles, for instance, have recently been explored for their antimicrobial properties [].
Various methods create nano-features on implant surfaces, classified as chemical (e.g., anodic oxidation, acid/oxidant treatments) or physical (e.g., plasma spray, blasting) []. Additionally, antimicrobial peptides like LL37 (a cationic antimicrobial peptide derived from human cathelicidin, primarily involved in host defense mechanisms by disrupting microbial membranes, modulating immune responses, and promoting wound healing) can be immobilized on implant surfaces to provide antimicrobial and angiogenic effects []. Both soluble and immobilized LL37 peptides are effective against Gram-positive and Gram-negative bacteria in 10% human serum, with the immobilized form showing lower cytotoxicity to endothelial cells.
Innovative materials that mimic natural bone tissue stimulate cell growth and the formation of new bone around the implant. Nanoparticles included in the composition of artificial bone help reduce the risk of implant rejection, promoting faster healing and integration. This is especially important for patients with bone deficiency or insufficient natural bone for stable implantation.
Nanophase materials offer promising solutions for treating bone deformities and defects. Two leading materials are nanophase hydroxyapatite and carbon. Nanophase hydroxyapatite promotes superior osteoblastic adhesion and is used in products such as NanOSSTM HA, Vitosso HA + tricalcium phosphate, and Ostim HA []. In contrast, carbon nanophase combines nanoscale properties with characteristics similar to natural hydroxyapatite, making it a strong candidate for future maxillofacial defect correction and implant applications [].
Using nanotechnology in implantology opens up new horizons for improving treatment outcomes. However, despite the numerous advantages, the implementation of these technologies is accompanied by specific challenges (Figure 4):
Modern strategies for the prevention and management of peri-implantitis, such as using implants with nanocoating and artificial bone incorporating nanoparticles, represent promising directions in modern dentistry. Careful understanding of the classification of periodontal diseases, to which peri-implantitis belongs, allows us to understand the pathogenesis of the disease better and develop effective methods of prevention and treatment. Further research and clinical trials of the latest technologies will help reduce the risk of implant rejection and improve patients' quality of life. Emerging strategies in treatment and material optimization offer new opportunities for instant diagnostic feedback, improved efficacy, and reduced side effects, which can significantly enhance patient rehabilitation.
Figures are original content created by authors.
The article was funded at the author’s own expense.
ML: Conceptualization, Investigation, Writing-original draft, Writing-review & editing, Supervision.
NT: Conceptualization, Investigation, Validation, Writing-original draft, Writing-review & editing.
Klinge B, Sanz M, Alcoforado G, Bienz SP, Cosyn J, De Bruyn H, Derks J, Figuero E, Gurzawska K, Heitz‐Mayfield L, Jung RE. Dental implant register: Summary and consensus statements of group 2. The 5th EAO Consensus Conference 2018. Clin Oral Implants Res. 2018 Oct;29(Suppl 18):157-159.
Ferreira SD, Martins CC, Amaral SA, Vieira TR, Albuquerque BN, Cota LOM, Esteves Lima RP, Costa FO. Periodontitis as a risk factor for peri-implantitis: Systematic review and meta-analysis of observational studies. J Dent. 2018 Dec;79:1-10. doi: 10.1016/j.jdent.2018.09.010. Epub 2018 Nov 2. PMID: 30391683.
Roccuzzo M, Layton DM, Roccuzzo A, Heitz-Mayfield LJ. Clinical outcomes of peri-implantitis treatment and supportive care: A systematic review. Clin Oral Implants Res. 2018 Oct;29 Suppl 16:331-350. doi: 10.1111/clr.13287. PMID: 30328195.
Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Clin Periodontol. 2018 Jun;45 Suppl 20:S246-S266. doi: 10.1111/jcpe.12954. PMID: 29926484.
Ravidà A, Galli M, Siqueira R, Saleh MHA, Galindo-Moreno P, Wang HL. Diagnosis of peri-implant status after peri-implantitis surgical treatment: Proposal of a new classification. J Periodontol. 2020 Dec;91(12):1553-1561. doi: 10.1002/JPER.20-0124. Epub 2020 Jun 23. PMID: 32449808.
World Health Organization. International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM). Geneva: WHO; 2016.
American Dental Association (ADA). Current Dental Terminology (CDT) 2024. Chicago: American Dental Association; 2024.
Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hämmerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018 Jun;45 Suppl 20:S286-S291. doi: 10.1111/jcpe.12957. PMID: 29926491.
Heitz-Mayfield LJA, Heitz F, Lang NP. Implant Disease Risk Assessment IDRA-a tool for preventing peri-implant disease. Clin Oral Implants Res. 2020 Apr;31(4):397-403. doi: 10.1111/clr.13585. Epub 2020 Feb 20. PMID: 32003037.
Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Clin Periodontol. 2018 Jun;45 Suppl 20:S237-S245. doi: 10.1111/jcpe.12953. PMID: 29926488.
Mangano FG, Admakin O, Lerner H, Mangano C. Artificial intelligence and augmented reality for guided implant surgery planning: A proof of concept. J Dent. 2023 Jun;133:104485. doi: 10.1016/j.jdent.2023.104485. Epub 2023 Mar 23. PMID: 36965859.
Jacobs R, Salmon B, Codari M, Hassan B, Bornstein MM. Cone beam computed tomography in implant dentistry: recommendations for clinical use. BMC Oral Health. 2018 May 15;18(1):88. doi: 10.1186/s12903-018-0523-5. PMID: 29764458; PMCID: PMC5952365.
Verma N, Kamboj G, Lata J, Singh P, Gowda S. Cone Beam Computed Tomography-Based Evaluation of the Accuracy of Implant Surgery with Conventional (Free Hand) Implant Placement vs Computer Fabricated 3D Guide Implant Placement. J Maxillofac Oral Surg. 2023 Dec;22(4):1115-1122. doi: 10.1007/s12663-023-01887-7. Epub 2023 Mar 27. PMID: 38105824; PMCID: PMC10719224.
Nulty A. A literature review on prosthetically designed guided implant placement and the factors influencing dental implant success. Br Dent J. 2024 Feb;236(3):169-180. doi: 10.1038/s41415-024-7050-3. Epub 2024 Feb 9. PMID: 38332076; PMCID: PMC10853061.
Ionescu A, Dodi A, Petcu LC, Nicolescu MI. Open Healing: A Minimally Invasive Protocol with Flapless Ridge Preservation in Implant Patients. Biology (Basel). 2022 Jan 14;11(1):142. doi: 10.3390/biology11010142. PMID: 35053140; PMCID: PMC8773332.
Saini RS, Bavabeedu SS, Quadri SA, Gurumurthy V, Kanji MA, Kuruniyan MS, Binduhayyim RIH, Avetisyan A, Heboyan A. Impact of 3D imaging techniques and virtual patients on the accuracy of planning and surgical placement of dental implants: A systematic review. Digit Health. 2024 May 7;10:20552076241253550. doi: 10.1177/20552076241253550. PMID: 38726220; PMCID: PMC11080757.
Ku JK, Lee J, Lee HJ, Yun PY, Kim YK. Accuracy of dental implant placement with computer-guided surgery: a retrospective cohort study. BMC Oral Health. 2022 Jan 16;22(1):8. doi: 10.1186/s12903-022-02046-z. PMID: 35034613; PMCID: PMC8762866.
Nogueira-Reis F, Morgan N, Suryani IR, Tabchoury CPM, Jacobs R. Full virtual patient generated by artificial intelligence-driven integrated segmentation of craniomaxillofacial structures from CBCT images. J Dent. 2024 Feb;141:104829. doi: 10.1016/j.jdent.2023.104829. Epub 2023 Dec 30. PMID: 38163456.
Matvijenko K, Borusevičius R. Comparison of dynamic navigation systems in dental implantology: a systematic literature review of in vitro studies. Int J Oral Maxillofac Surg. 2025 Feb 19.
de Avila ED, van Oirschot BA, van den Beucken JJJP. Biomaterial-based possibilities for managing peri-implantitis. J Periodontal Res. 2020 Apr;55(2):165-173. doi: 10.1111/jre.12707. Epub 2019 Oct 22. PMID: 31638267; PMCID: PMC7154698.
Costa FO, Costa AM, Ferreira SD, Lima RPE, Pereira GHM, Cyrino RM, Oliveira AMSD, Oliveira PAD, Cota LOM. Long-term impact of patients' compliance to peri-implant maintenance therapy on the incidence of peri-implant diseases: An 11-year prospective follow-up clinical study. Clin Implant Dent Relat Res. 2023 Apr;25(2):303-312. doi: 10.1111/cid.13169. Epub 2022 Dec 15. PMID: 36519351.
Sun TC, Chen CJ, Gallucci GO. Prevention and management of peri-implant disease. Clin Implant Dent Relat Res. 2023 Aug;25(4):752-766. doi: 10.1111/cid.13206. Epub 2023 Apr 12. PMID: 37042296.
Diaz P, Gonzalo E, Villagra LJG, Miegimolle B, Suarez MJ. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health. 2022 Oct 19;22(1):449. doi: 10.1186/s12903-022-02493-8. PMID: 36261829; PMCID: PMC9583568.
Chouirfa H, Bouloussa H, Migonney V, Falentin-Daudré C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019 Jan 1;83:37-54. doi: 10.1016/j.actbio.2018.10.036. Epub 2018 Oct 26. PMID: 30541702.
Rasouli R, Barhoum A, Uludag H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning, and functionalization for improved performance. Biomater Sci. 2018;6(6):1312–1338.
Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning, and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338.
Comune M, Rai A, Palma P, Tonda-Turo C, Ferreira L. Antimicrobial and pro-angiogenic properties of soluble and nanoparticle-immobilized LL37 peptides. Biomater Sci. 2021;9(22):8153–8159.
Nandagopal N, Usha M, Sreejith S, Rajan S. A clinical review of nanotechnology in maxillofacial practice. J Oral Res Rev. 2021;13(3):149–160.
Pushpalatha C, Gayathri VS, Sowmya SV, Augustine D, Alamoudi A, Zidane B, Hassan Mohammad Albar N, Bhandi S. Nanohydroxyapatite in dentistry: A comprehensive review. Saudi Dent J. 2023 Sep;35(6):741-752. doi: 10.1016/j.sdentj.2023.05.018. Epub 2023 Jun 7. PMID: 37817794; PMCID: PMC10562112.
Levkiv M, Tverdokhlib N. Innovative Strategies in the Prevention and Treatment of Peri-implantitis. IgMin Res. March 09, 2025; 3(4): 155-159. IgMin ID: igmin295; DOI:10.61927/igmin295; Available at: igmin.link/p295
任何您分享以下链接的人都可以阅读此内容:
1Dental Therapy Department, I. Horbachevsky Ternopil National Medical University, Ternopil, 46003, Ukraine
2Dental Surgery Department, I. Horbachevsky Ternopil National Medical University, Ternopil, 46003, Ukraine
Address Correspondence:
Mariana Levkiv, Dental Therapy Department, I. Horbachevsky Ternopil National Medical University, Ternopil, 46003, Ukraine, Email: [email protected]
How to cite this article:
Levkiv M, Tverdokhlib N. Innovative Strategies in the Prevention and Treatment of Peri-implantitis. IgMin Res. March 09, 2025; 3(4): 155-159. IgMin ID: igmin295; DOI:10.61927/igmin295; Available at: igmin.link/p295
Copyright: © 2025 Levkiv M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
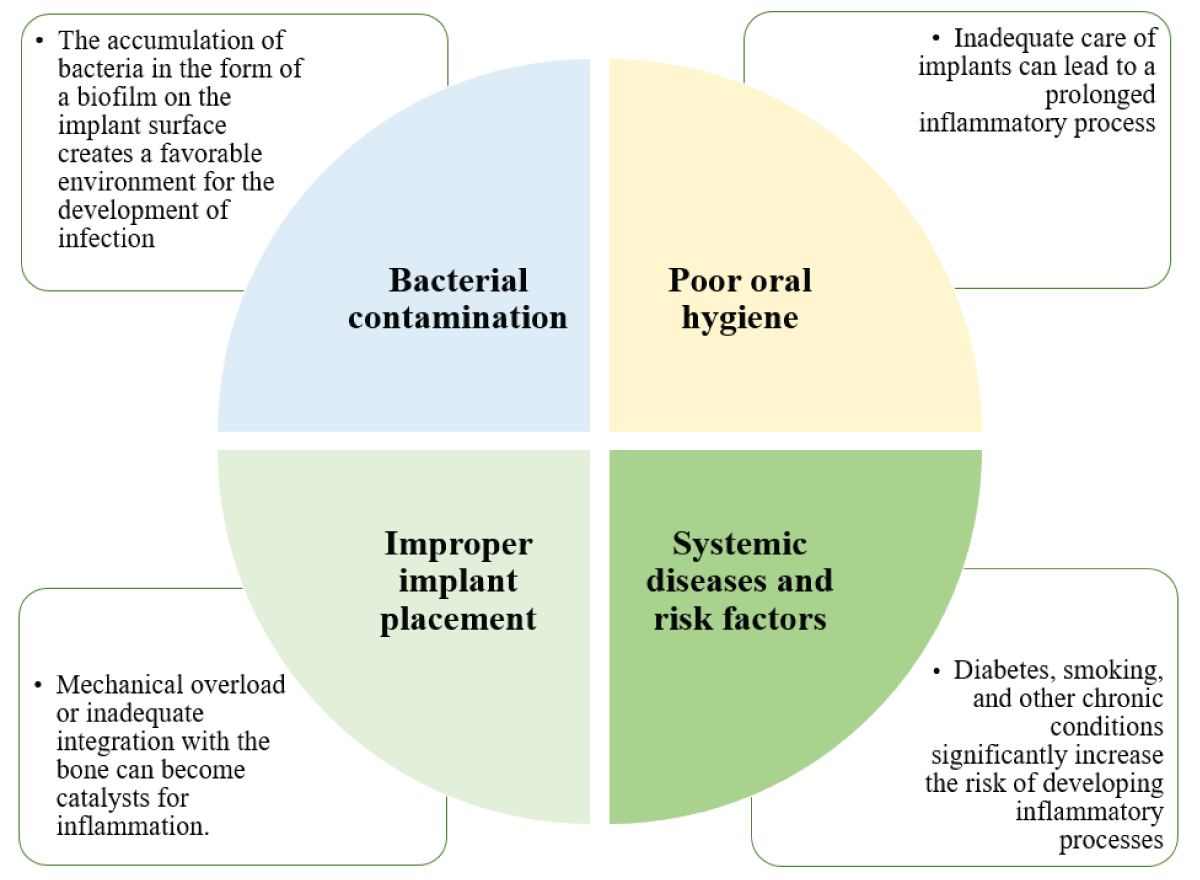 Figure 1: Main reasons for peri-implantitis....
Figure 1: Main reasons for peri-implantitis....
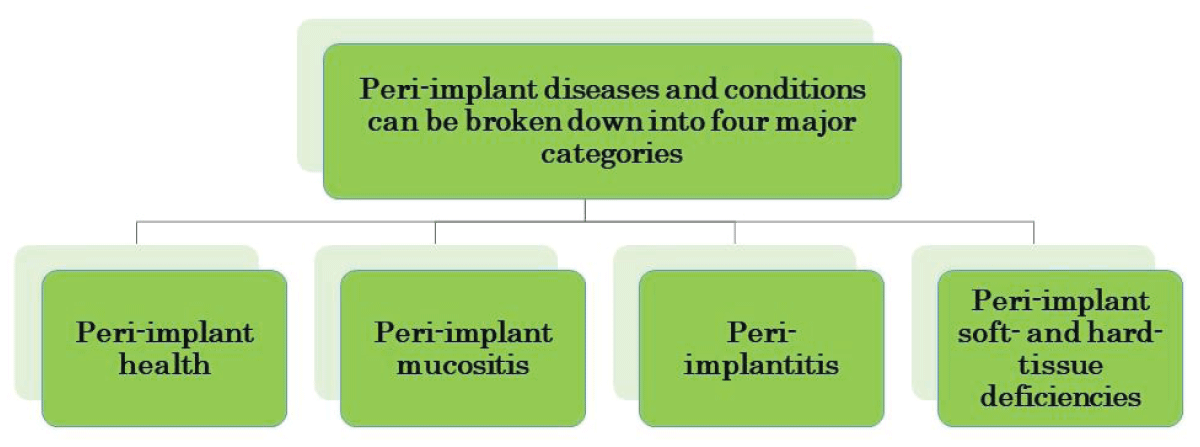 Figure 2: Categories of peri-implant diseases and conditions...
Figure 2: Categories of peri-implant diseases and conditions...
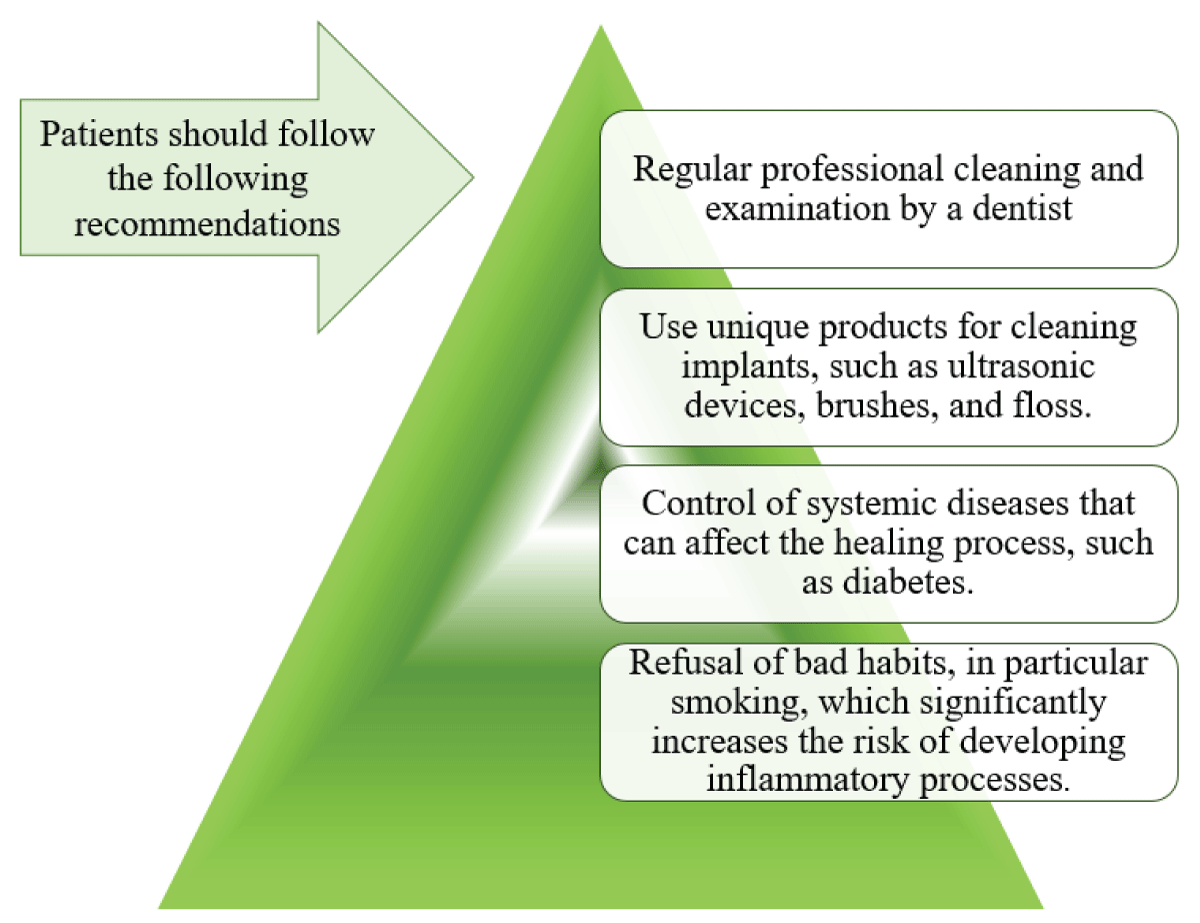 Figure 3: The basis of prevention of peri-implantitis....
Figure 3: The basis of prevention of peri-implantitis....
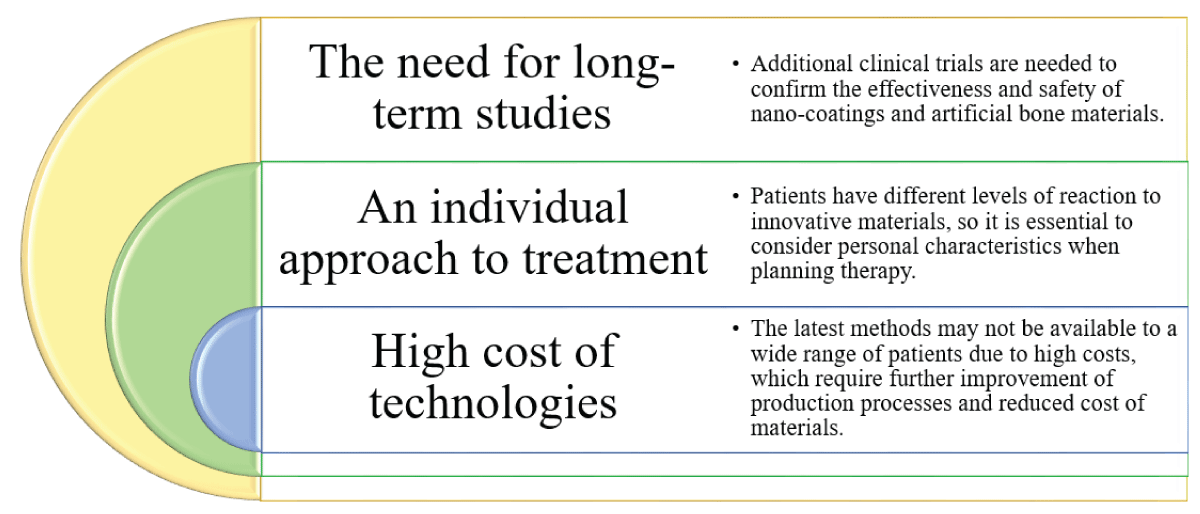 Figure 4: Challenges and limitations of nanotechnology in de...
Figure 4: Challenges and limitations of nanotechnology in de...
Klinge B, Sanz M, Alcoforado G, Bienz SP, Cosyn J, De Bruyn H, Derks J, Figuero E, Gurzawska K, Heitz‐Mayfield L, Jung RE. Dental implant register: Summary and consensus statements of group 2. The 5th EAO Consensus Conference 2018. Clin Oral Implants Res. 2018 Oct;29(Suppl 18):157-159.
Ferreira SD, Martins CC, Amaral SA, Vieira TR, Albuquerque BN, Cota LOM, Esteves Lima RP, Costa FO. Periodontitis as a risk factor for peri-implantitis: Systematic review and meta-analysis of observational studies. J Dent. 2018 Dec;79:1-10. doi: 10.1016/j.jdent.2018.09.010. Epub 2018 Nov 2. PMID: 30391683.
Roccuzzo M, Layton DM, Roccuzzo A, Heitz-Mayfield LJ. Clinical outcomes of peri-implantitis treatment and supportive care: A systematic review. Clin Oral Implants Res. 2018 Oct;29 Suppl 16:331-350. doi: 10.1111/clr.13287. PMID: 30328195.
Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Clin Periodontol. 2018 Jun;45 Suppl 20:S246-S266. doi: 10.1111/jcpe.12954. PMID: 29926484.
Ravidà A, Galli M, Siqueira R, Saleh MHA, Galindo-Moreno P, Wang HL. Diagnosis of peri-implant status after peri-implantitis surgical treatment: Proposal of a new classification. J Periodontol. 2020 Dec;91(12):1553-1561. doi: 10.1002/JPER.20-0124. Epub 2020 Jun 23. PMID: 32449808.
World Health Organization. International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM). Geneva: WHO; 2016.
American Dental Association (ADA). Current Dental Terminology (CDT) 2024. Chicago: American Dental Association; 2024.
Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hämmerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018 Jun;45 Suppl 20:S286-S291. doi: 10.1111/jcpe.12957. PMID: 29926491.
Heitz-Mayfield LJA, Heitz F, Lang NP. Implant Disease Risk Assessment IDRA-a tool for preventing peri-implant disease. Clin Oral Implants Res. 2020 Apr;31(4):397-403. doi: 10.1111/clr.13585. Epub 2020 Feb 20. PMID: 32003037.
Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Clin Periodontol. 2018 Jun;45 Suppl 20:S237-S245. doi: 10.1111/jcpe.12953. PMID: 29926488.
Mangano FG, Admakin O, Lerner H, Mangano C. Artificial intelligence and augmented reality for guided implant surgery planning: A proof of concept. J Dent. 2023 Jun;133:104485. doi: 10.1016/j.jdent.2023.104485. Epub 2023 Mar 23. PMID: 36965859.
Jacobs R, Salmon B, Codari M, Hassan B, Bornstein MM. Cone beam computed tomography in implant dentistry: recommendations for clinical use. BMC Oral Health. 2018 May 15;18(1):88. doi: 10.1186/s12903-018-0523-5. PMID: 29764458; PMCID: PMC5952365.
Verma N, Kamboj G, Lata J, Singh P, Gowda S. Cone Beam Computed Tomography-Based Evaluation of the Accuracy of Implant Surgery with Conventional (Free Hand) Implant Placement vs Computer Fabricated 3D Guide Implant Placement. J Maxillofac Oral Surg. 2023 Dec;22(4):1115-1122. doi: 10.1007/s12663-023-01887-7. Epub 2023 Mar 27. PMID: 38105824; PMCID: PMC10719224.
Nulty A. A literature review on prosthetically designed guided implant placement and the factors influencing dental implant success. Br Dent J. 2024 Feb;236(3):169-180. doi: 10.1038/s41415-024-7050-3. Epub 2024 Feb 9. PMID: 38332076; PMCID: PMC10853061.
Ionescu A, Dodi A, Petcu LC, Nicolescu MI. Open Healing: A Minimally Invasive Protocol with Flapless Ridge Preservation in Implant Patients. Biology (Basel). 2022 Jan 14;11(1):142. doi: 10.3390/biology11010142. PMID: 35053140; PMCID: PMC8773332.
Saini RS, Bavabeedu SS, Quadri SA, Gurumurthy V, Kanji MA, Kuruniyan MS, Binduhayyim RIH, Avetisyan A, Heboyan A. Impact of 3D imaging techniques and virtual patients on the accuracy of planning and surgical placement of dental implants: A systematic review. Digit Health. 2024 May 7;10:20552076241253550. doi: 10.1177/20552076241253550. PMID: 38726220; PMCID: PMC11080757.
Ku JK, Lee J, Lee HJ, Yun PY, Kim YK. Accuracy of dental implant placement with computer-guided surgery: a retrospective cohort study. BMC Oral Health. 2022 Jan 16;22(1):8. doi: 10.1186/s12903-022-02046-z. PMID: 35034613; PMCID: PMC8762866.
Nogueira-Reis F, Morgan N, Suryani IR, Tabchoury CPM, Jacobs R. Full virtual patient generated by artificial intelligence-driven integrated segmentation of craniomaxillofacial structures from CBCT images. J Dent. 2024 Feb;141:104829. doi: 10.1016/j.jdent.2023.104829. Epub 2023 Dec 30. PMID: 38163456.
Matvijenko K, Borusevičius R. Comparison of dynamic navigation systems in dental implantology: a systematic literature review of in vitro studies. Int J Oral Maxillofac Surg. 2025 Feb 19.
de Avila ED, van Oirschot BA, van den Beucken JJJP. Biomaterial-based possibilities for managing peri-implantitis. J Periodontal Res. 2020 Apr;55(2):165-173. doi: 10.1111/jre.12707. Epub 2019 Oct 22. PMID: 31638267; PMCID: PMC7154698.
Costa FO, Costa AM, Ferreira SD, Lima RPE, Pereira GHM, Cyrino RM, Oliveira AMSD, Oliveira PAD, Cota LOM. Long-term impact of patients' compliance to peri-implant maintenance therapy on the incidence of peri-implant diseases: An 11-year prospective follow-up clinical study. Clin Implant Dent Relat Res. 2023 Apr;25(2):303-312. doi: 10.1111/cid.13169. Epub 2022 Dec 15. PMID: 36519351.
Sun TC, Chen CJ, Gallucci GO. Prevention and management of peri-implant disease. Clin Implant Dent Relat Res. 2023 Aug;25(4):752-766. doi: 10.1111/cid.13206. Epub 2023 Apr 12. PMID: 37042296.
Diaz P, Gonzalo E, Villagra LJG, Miegimolle B, Suarez MJ. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health. 2022 Oct 19;22(1):449. doi: 10.1186/s12903-022-02493-8. PMID: 36261829; PMCID: PMC9583568.
Chouirfa H, Bouloussa H, Migonney V, Falentin-Daudré C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019 Jan 1;83:37-54. doi: 10.1016/j.actbio.2018.10.036. Epub 2018 Oct 26. PMID: 30541702.
Rasouli R, Barhoum A, Uludag H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning, and functionalization for improved performance. Biomater Sci. 2018;6(6):1312–1338.
Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning, and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338.
Comune M, Rai A, Palma P, Tonda-Turo C, Ferreira L. Antimicrobial and pro-angiogenic properties of soluble and nanoparticle-immobilized LL37 peptides. Biomater Sci. 2021;9(22):8153–8159.
Nandagopal N, Usha M, Sreejith S, Rajan S. A clinical review of nanotechnology in maxillofacial practice. J Oral Res Rev. 2021;13(3):149–160.
Pushpalatha C, Gayathri VS, Sowmya SV, Augustine D, Alamoudi A, Zidane B, Hassan Mohammad Albar N, Bhandi S. Nanohydroxyapatite in dentistry: A comprehensive review. Saudi Dent J. 2023 Sep;35(6):741-752. doi: 10.1016/j.sdentj.2023.05.018. Epub 2023 Jun 7. PMID: 37817794; PMCID: PMC10562112.